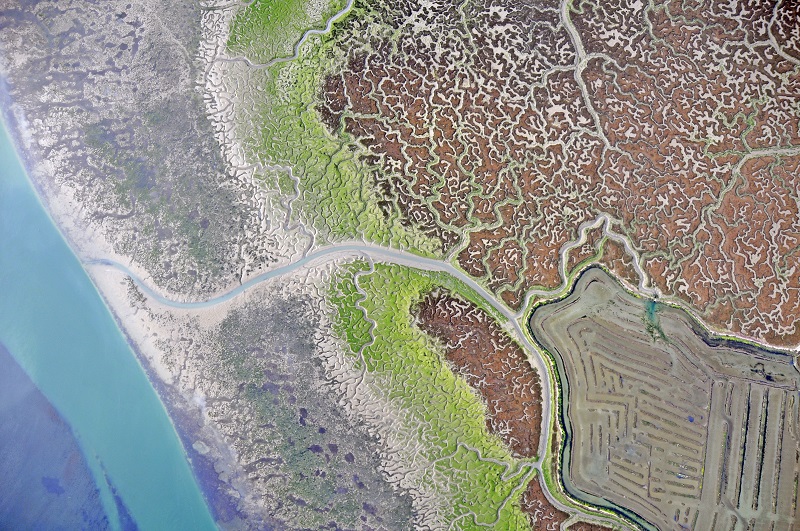
University of Cádiz Installs Spain’s First Eddy Covariance Tower to Study Blue Carbon in Tidal Marshes
The University of Cádiz (UCA) has taken a pioneering step in climate change research with the installation of Spain’s first Eddy Covariance tower dedicated to studying blue carbon sequestration in intertidal ecosystems.

This cutting-edge infrastructure is part of the RICAS project (Restoration and Rewilding of Saltmarshes as Nature-Based Solutions against Climate Change), funded by the European Union’s Recovery and Resilience Facility (NextGenerationEU). The project is led by Dr. Alfonso Corzo and Dr. Sokratis Papaspyrou from UCA’s Department of Biology.

Marine Rewilding: Restoring to Recover Ecological Functions
The saltmarshes and salterns of Cádiz Bay, like many coastal areas in Europe, have undergone centuries of human transformation — from artisanal and industrial saltworks to aquaculture and agricultural use.

The rewilding approach seeks to reverse this degradation by restoring the original ecological functions of these ecosystems. Within the framework of the EU Nature Restoration Law, such actions are recognized as Nature-Based Solutions (NBS), essential to halt biodiversity loss and address the impacts of sea-level rise, coastal erosion, and climate change.
A One-of-a-Kind Infrastructure
The new Eddy Covariance tower will enable real-time measurement of carbon, water, and energy fluxes between the marsh surface and the atmosphere. Using this high-precision technology, researchers will quantify the net carbon balance of different marsh types — natural, abandoned, or under restoration — with unprecedented detail in Spain.

These continuous observations will help reduce existing uncertainties in estimating blue carbon sequestration, providing key insights into how tidal marshes contribute to climate change mitigation.

Science, Environment, and a Sustainable Future
The RICAS project combines biogeochemistry, remote sensing, and numerical modelling in an interdisciplinary framework. A team of experts in coastal ecology, physical oceanography, and data engineering is working together to deliver scientific results with environmental and socio-economic impact.

The project aims to:
- Improve quantification and modelling of carbon fluxes in tidal marshes.
- Develop management and restoration tools for the Bahía de Cádiz Natural Park and other intertidal zones.
- Support the creation of voluntary blue carbon markets, opening new funding opportunities for wetland conservation.
- Align local and national policies with EU and United Nations goals on biodiversity, climate neutrality, and ecosystem restoration.
“This tower marks a milestone for coastal research in Spain. For the first time, we’ll be able to track the carbon pulse of our marshes, understand their response to climate change, and design effective restoration strategies,” says Dr. Alfonso Corzo, principal investigator of the project.
MEB-LAB Deploys High-Tech Oceanographic Buoy for Advanced Monitoring of the Bay of Cádiz
The University of Cádiz (UCA) has today installed a cutting-edge oceanographic buoy in the inner basin of the Bay of Cádiz. This marks a crucial milestone for the project “Multiscalar monitoring of carbon sequestration, biodiversity, and climate change in coastal marshes,” an initiative led by researchers Sokratis Papaspyrou and Alfonso Corzo. The project is part of the Marine Science Complementary Plan and the Recovery, Transformation, and Resilience Plan, which provide the funding for this endeavor.


The buoy is equipped with a multiparametric probe that will continuously measure various water column properties with high resolution. These include temperature, conductivity, pH, dissolved oxygen, chlorophyll (via fluorescence), turbidity, and current speed and direction. This real-time data will be fundamental to understanding the effects of climate change and extreme events—such as storms and heatwaves—on estuarine ecosystems.
Importance for Fisheries, Shellfishing, and Coastal Management
The continuous monitoring of water quality and productivity in the Bay of Cádiz is of vital importance to the local fishing and shellfishing sectors. Parameters such as dissolved oxygen, temperature, and chlorophyll are key indicators of marine ecosystem health and food availability for various species. Optimal water quality and high primary productivity (linked to chlorophyll levels) are essential for the growth and survival of fish and shellfish populations, ensuring the sustainability of these deeply rooted economic activities in the region.
Furthermore, combining turbidity and chlorophyll data with current speed and direction (via the current meter) will provide crucial information for estimating material transport within the bay. This is essential for understanding sediment and nutrient dynamics. With this data, researchers will be able to predict the impact of sea-level rise on the coastline and system productivity, allowing for the development of more effective coastal management strategies and climate change adaptation.
A Step Toward the MLab-Bahía
This project falls under the “Observation and monitoring of the marine and coastal environment” line of action and lays the foundation for the creation of the Coastal Laboratory for Advanced Multiscalar Monitoring of the Bay of Cádiz (MLab-Bahía). The goal of MLab-Bahía is to implement a comprehensive observation platform spanning from the water to the upper limits of the salt marsh, integrating various tools and technologies to track the marine ecosystem across all dimensions.

The information gathered by the buoy will not only enrich scientific knowledge regarding carbon flows and biodiversity in intertidal zones but will also be highly valuable for:
- The Bay of Cádiz Natural Park management office.
- The Atlantic Coast Directorate.
- The Port Authority of the Bay of Cádiz.
- The Regional Ministry of the Environment (Junta de Andalucía) and local town councils.
This data will help identify areas of special interest for protection, restoration, and the improvement of management practices within this protected area. The installation of this multiparametric buoy is a significant step toward strengthening the resilience of marine ecosystems and creating surveillance networks, positioning the Bay of Cádiz as a benchmark in the observation and study of coastal marshes.

MEBL at the BlueZoneForum-Innovazul congress
On November 20, 21 and 22, the third edition of Innovazul will be held at the Congress Palace in Cádiz. This edition is organized in collaboration with the Zona Franca of Cadiz integrating two types of events in one: BlueZoneForum_INNOVAZUL 2024.
Our lab is there presenting a stand of our work related to the project Lab Bahia (PCM0030). We will also be presenting instrumentation, some of which we have developed ourselves, that is used in this and other closely related projects.
Looking forward to all the talks and interactions.

Lab-Bahia: Postdoc offer to study the biogeochemical fluxes in intertidal ecosystems

We offer of postdoctoral contract until 31/12/2025, with the possibility of an extension (conditions apply). The applicant is expected to contribute to research on the study of primary production, biogeochemical fluxes and carbon sequestration and biodiversity patterns in response to climate change in the project “Multiscale monitoring of carbon sequestration, biodiversity and climate change in coastal marshes” (Lab-Bahia). Lab-Bahia is a project funded by the Supplementary Marine Science Plan and the Recovery, Transformation and Resilience Plan of the Spanish Ministry of Science and Innovation.

What?
We are looking for a postdoctoral researcher to assist with the following tasks:
Contribute to research on the study of primary production, biogeochemical fluxes and carbon sequestration and biodiversity patterns in response to climate change.
– Analyse the information obtained by remote sensing techniques (drones and satellites) and perform the scaling and mapping of project variables.
– Carry out in situ measurements with field instruments for the measurement of biogeochemical variables and the biogeochemical variables and validation of remotely sensed data.
– Data processing and statistical analysis of environmental variables for the determination of spatial patterns of the spatial patterns of photosynthetic communities and primary production in intertidal zones.
– Extract data on biogeochemical, environmental and climatic variables from different sources, organization and sources, organization and preparation for analysis and incorporation into databases.
– Support the development of biogeochemical models in aquatic systems related to primary production in sediment and production in sediment and water, gas and nutrient exchanges at the sediment-water and sediment-air interface, and microbial processes.

When?
The candidate should start as soon as possible (earliest expected date is early January, which depends on the selection process).
Where?
The selected candidate will join the Microbial Ecology and Biogeochemistry Group of the Department of Biology of the University of Cadiz, under the supervision of Sokratis Papaspyrou.
Preferable skills
– Experience in the measurement of sediment-water and sediment-air fluxes using benthic chambers with IRGA, FTIR, etc. or Eddy covariance towers.
– Experience in spatial ecology of communities, mainly marine photosynthetic communities, and carbon cycle biogeochemistry.
– Experience in Geographic Information Systems (ArcGis, QGis) and advanced programming (ArcPy, Python, Python).
– Experience in the use of statistical tools, for spatial and time series analysis.
– Experience in other techniques related to Microbial Ecology and Biogeochemistry in wetlands and marshes will be highly valued.
– Good knowledge of written and spoken English (scientific level).
How to apply?
The contract offer is published in Spanish in the University of Cádiz website (https://personal.uca.es/convocatorias-de-capitulo-vi-2024/). The call will open from 14th to 23rd November 2024. The specific information is in ANEXOS-nOVIEMBRE-2024 (Anexo 1 in Spanish, English translation here).
https://personal.uca.es/convocatorias-de-capitulo-vi-2024/
Call documents: Annex Calls November 2024
If you require more info or help to apply, please send an email to sokratis.papaspyrou@uca.es. Please, send an email with your CV (any format) and a cover letter before applying for the position. Two reference letters should also be sent by referees to the same email, maximum two weeks after the closing date.
Sandra’s stay at SDU
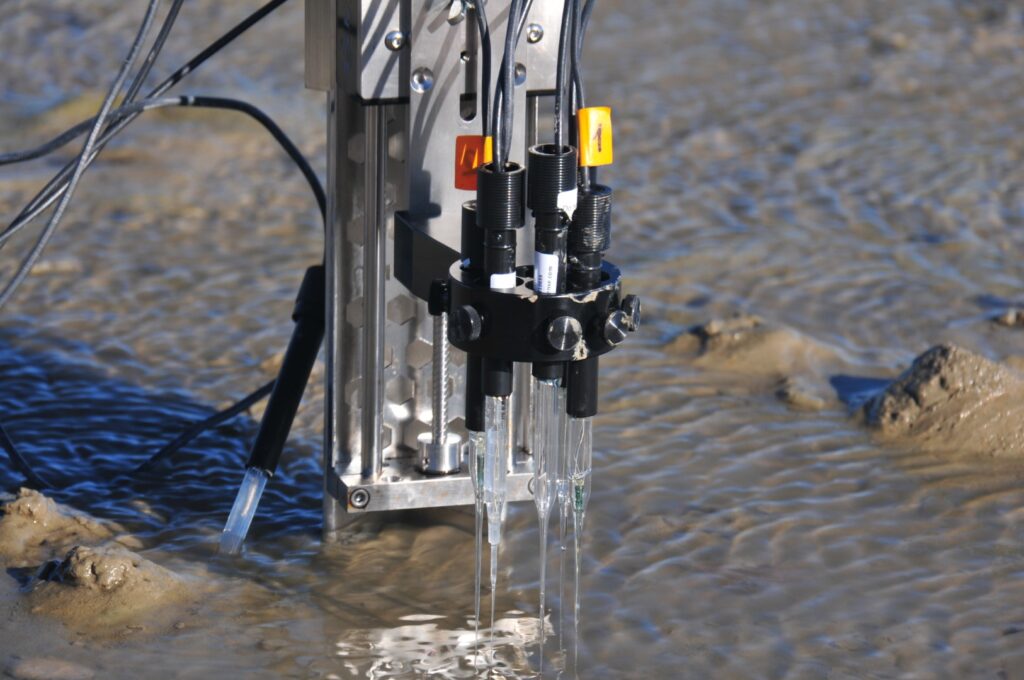
After five months in Odense (Denmark), I have completed my stay at the University of Southern Denmark (SDU), where, in addition to learning how Aquatic Eddy Covariance (AEC) works, I conducted two experiments that provide key information on its application in intertidal zones. Although AEC is an innovative method for measuring O2 fluxes and studying various dynamics in aquatic ecosystems, its use remains limited due to its complexity. Internationally, only a few laboratories have adopted this technique, and in Spain, only one research institution has implemented it to date.

Aquatic Eddy covariance. A bit of an introduction….
The AEC is a non-invasive method that allows the measurement of benthic biogeochemical fluxes with a high resolution, during days or weeks continuously. This method is a relatively new technique (Berg et al., 2003). Over the last two decades, the quality of benthic O2 flux measurements by AEC has improved by incorporating new rapid response systems that measure O2. Due to their complexity, O2 measurement systems used for AEC must undergo rigorous testing under well-defined laboratory and field conditions to assess performance, reliability and potential limitations (Berg et al., 2016; Chipman et al., 2012; McGinnis et al., 2011).
An example of a new O2 measurement system is the AquapHOx-LX logger (PyroScience, GmbH; referred to as AquapHOx). It is an optical submersible meter, which has been designed to perform high resolution O2 measurements underwater, making it a suitable option for collecting robust AEC data.

In intertidal zones, the use of the AquapHOx system can present challenges due to temperature variations between the sediment and the water column. This can occur because, during low tide, the sediment is directly exposed to solar radiation, which causes significant heating of the sediment surface. When the tide rises and the sediment returns underwater, the temperature difference can generate heat fluxes from the seabed to the water column. These thermal fluctuations, however small, can affect the O2 signal. This potential error in the O₂ signal can alter the interpretation of biogeochemical fluxes in these areas.

Since AEC is a relatively new technique (Berg et al., 2003), there are hardly any studies in intertidal zones (Volaric et al., 2020) that quantify the importance of heat fluxes between the sediment and the water column. Therefore, it is necessary to test:
- how much the O2 signal is affected by temperature, i.e., what percentage of the oxygen fluctuations are driven by natural oxygen changes versus temperature effects, and
- how long it takes for the sediment surface and water to equilibrate in temperature.
To verify this, we performed two short experiments. The first consisted of quantifying the effect of temperature on the O2 signal; the second consisted in quantifying the time it takes for the sediment surface temperature to equilibrate with the water column.
Test 1. Evaluating the response of the O2 signal to temperature changes.
The sensitivity of the O₂ sensor to thermal variations was evaluated following the procedure described by Granville et al. (2023).
Test 2. Quantifying the time, it takes for the sediment surface temperature to equilibrate with the water column after the sediment surface was exposed to high temperature
This experiment aimed to measure how long sediment takes to reach equilibrium with the water column after being exposed to high temperatures, simulating low tide conditions. Additionally, it also examined whether sediment color affects heat absorption.
To do that, five sediment cores of different color were collected from Odense Fjord, Denmark, acclimatized for a day. After that, we performed different temperature profiles, with and without a water column, to obtain initial conditions.
The sediment surfaces were then exposed to high light intensity (simulating solar radiation) for 2 hours without water. Afterward, temperature profiles were measured every 20 minutes using a temperature probe until the temperatures returned to initial levels. The process was then repeated for each core in the presence of a water column.
After these experiment, we can conclude that, although, the sediment surface may heat up more in the absence of the water column and in darker sediments, the recovery time showed no observable differences. Furthermore, considering that the temperature of the water column and of the uppermost layers of the sediment quickly returned to their initial conditions, it can be concluded that AquapHOx can be used in intertidal zones.
Irene at WHOI
Irene’s doctoral thesis investigates the effects of decreasing oxygen conditions on microbial communities through the aerobic respiration kinetics and how these communities adapt at the oxic-anoxic interfaces. As a part of her PhD work, Irene has participated in two oceanographic expeditions to oxygen minimum zones (OMZs) in the North and South Pacific Ocean. During these campaigns aboard scientific research vessels, she performed on-site experiments and collected samples for molecular biology analysis, aiming to understand microbial behaviour at the genetic level.
Currently, Irene is undertaking a predoctoral research stay at the prestigious Woods Hole Oceanographic Institution (Massachusetts, USA) from September 20 to December 20, 2024, under the supervision of Maria G. Pachiadaki, an expert in molecular biology, particularly in metagenomics and metatranscriptomics of marine environments. During this stay, Irene is conducting DNA and RNA extractions to describe microbial communities, as well as identifying key genes and enzymes involved in their adaptation to decreasing oxygen conditions. This research sheds light on how microorganisms adjust to oxygen-depleted oceans, where critical biogeochemical cycles and functioning of the world’s oceans depend heavily on microbial processes.
MULTI-FLUX: PhD offer to study the primary production of benthic communities in shallow coastal areas and the effect of climate change
PhD offer to study the primary production of benthic communities and the impact of Extreme Climatic Events, such as heat-waves and wind storms, on shallow coastal areas, focusing on the impacts on the biological community and biogeochemical functioning .
What?
We are looking for a motivated candidate to pursue a doctoral thesis in microbial ecology and biogeochemistry of coastal ecosystems. The project will be supported by the MULTIFLUX project, which aims to quantify in an integrated manner the effect of heat waves and wind storms, affecting temperature and sediment resuspension conditions, on the biogeochemical functioning of a shallow coastal ecosystem.

The candidate will use a combination of laboratory experiments, field measurements, and aerial data (satellite, drones…) to study the spatial and temporal variability benthic communities and its contribution to the primary production of the system and evaluate the effect of changes in external forcing by temperature and resuspension/hydrodynamics under normal and extreme conditions on benthic-pelagic coupling and carbon flux throughout the system. Strong focus will be given in the use of benthic chambers and the Aquatic Eddy Covariance technique, in collaboration with our colleagues in SDU (DK). For more information check the project’s webpage here.
When?
It is expected for the PhD to start in January 2025 and will last 4 years, with no possibility of an extension. Depending on the date of the PhD defense, there is the possibility of a postdoc contract within this 4 year period (all contracts included).

Where?
The PhD will be developed in the Microbial Ecology and Biogeochemistry Group of the Department of Biology of the University of Cadiz, under the supervision of Sokratis Papaspyrou and Irene Laiz.
Requirements
- The candidate must have obtained a bachelor’s degree on Marine or Environmental Sciences, Biology or similar and a Master’s degree preferably also in the same topics.
- The candidates must request to be pre-registered in a Doctoral Program in Marine Science and Technology at the University of Cadiz (https://posgrado.uca.es/doctor/)
- Be highly motivated and committed to develop a scientific career.
- Have some experience on biogeochemistry, aquatic ecology, microbiology, remote sensing, environmental chemistry or similar.
- Have experience on scientific reading and writing.
- Have a high level of written and spoken English.
How to apply?
Applications are open from from October 23rd to November 6th, 2024 (23:59) on the website: https://ugi.uca.es/convocatoria-formacion-personal-investigador-ministerio-de-ciencia-2024/
Please read the call carefully. If in doubt about any aspect (official call is in Spanish), please let us know.
Applications
- Send the application electronically through the following link: https://sedelectronica.uca.es/procedimientos/?proc=474
- IMPORTANT: In order to get access to the system, the applicant must send an e-mail to rrhh.investigacion@uca.es indicating his/her interest in submitting an application mentioning the call and project. Please do this well in advance before the deadline, as this is not an automated system. If you experience delays, please email us.
- Project Reference: PID2023-146617OB-I00 “Monitorización multiescalar de flujos biogeoquímicos y producción primaria en sistemas costeros someros: el efecto del forzamiento físico y del cambio climático”
- Only a single application is allowed per candidate.
- Documentation to be attached:
- Documentation certifying pre-admission to the PhD Program by filling in this form. As this has to be signed by the director of the school, please send it to us, we will do it for you and we will send it back to you.
- Curriculum vitae, using this template: CV
- Academic certificates issued by the universities where the Bachelor’s and Master’s degrees were obtained: the grades obtained, the scale used, and the date of graduation must appear. For the bachelor’s a full transcript should be provided. If the certificate is in a language other than Spanish or English, an official translation should be included. It is recommended that you check and include in the CV the corresponding scales based on these tables. https://www.universidades.gob.es/wp-content/uploads/2022/12/AnexoII-equivalencias.pdf and https://www.universidades.gob.es/wp-content/uploads/2022/12/AnexoI_escalas_1.pdf
- Copy of valid passport in case of foreign, non Spanish resident, citizens.
- Any other documents specified in the call. Please read it carefully.
- Make sure you upload a pdf copy of all certificates of degrees, courses, languages, employment contracts etc. to back the CV claims. The documentation supporting the merits to be evaluated must be submitted in a single PDF file with a maximum size of 15 MB. If no certificates are included, according to Spanish law, the merits cannot be considered.
- Online interviews will be arranged, if all the paperwork is in order and HR forwards the application to us, during November.
Please, send an email with your CV (any format) and a cover letter to sokratis.papaspyrou@uca.es before applying for the position. Two reference letters should also be sent by referees to the same email, by November 15th, 2024.
Only applications sent by the official channel will be considered by HR. Sending an email to us is not enough.
Please, be informed that the net salary for the employee after tax and social security contributions for such positions in Spain is approx. 1200€/mo (fixed by the Ministry of Science and Research), plus an extra pay in June and December (check this table). This salary is lower than in other European countries, however the cost of living is also lower. Here is a comparison of the cost of living between Brussels and Cádiz for indicative purposes only.
The effect of an invasive algae on the beach biogeochemistry
We have successfully completed the second year of field samplings of the project “Beach biodiversity and the ecological role of the invasive species Rugulopteryx okamurae in beach ecosystem functioning” Project Excel_01050, lead by Ivan Franco Rodíl.
During the second year, we contributed in measuring the bacterial diversity and leaching of dissolved organic carbon associated with accumulation of the invasive algae on different beaches of the coast of Cádiz.
The samples are currently being analysed but they show already how the organic matter of the beach racks alters the microbial benthic community along the beach transect. Hopefully the results will contribute to the management of this environmental issue that affects the local economy (fisheries, tourism).
Effects of heatwaves on the physiology of benthic microalgae
Andalusia has a high diversity of wetlands. These include intertidal shallow systems, which are important both ecologically and economically, given that the microalgae that grow at the sediment surface are an important source of food for higher-level organisms such as fish, oysters etc.

The microphytobenthos (MPB) is the photosynthetic community living on the sediment surface. They play an important role in shallow estuaries as they can be responsible for a large part of the primary production. This community is dominated by diatoms (algae covered in a silicate casing, called the frustule) and can achieve very high densities giving a golden colour at the sediment surface.
In order to deal with the highly fluctuating environments conditions that characterize the intertidal areas, such as light, temperature, tide cycle and seasonal variations, diatoms have developed several strategies. For example, they have the capacity to migrate vertically during the day to adapt to the light conditions and tidal variation.
Marika Mecca, one of our PhD students, is trying to understand the effects of climate change on the physiology and behavior of these benthic microalgae. Under normal conditions, the sediment surface undergoes large changes in temperature between night and day, under water (immersion) or exposed to the air (emersion), and between seasons. Thus, under a climate change scenario where heatwaves are more frequent, the shifts in temperature will be even larger and the effects on the physiology of the microalgae can be detrimental.
To study these effects, Marika uses various instruments and sensors in the lab, such as oxygen microsensors to measure the amount of oxygen produced, temperature sensors to measure the variations vertically, reflectance sensors to detect the migration rhythms etc.
The principal area of study is the Guadalquivir River. Once the cores are collected in the field, back at the laboratory these sediments are subjected to different conditions simulating the changes observed in the field under normal and extreme weather conditions.
The results show an effect of prolonged high temperatures on the migration rhythm of the microalgae (avoidance of the sediment surface), although differences in total net metabolism are less probably due to a stimulation of rates by the higher temperature. These results were presented at the ASLO 2023 conference in Mallorca.
Sandra’s research stay in SDU
From 1 May until 1 October 2024, Sandra Rizzo, one of the pre-doctoral students from our laboratory, is visiting the University of Southern Denmark (SDU, Odense) on an international stay to learn the use of the novel Aquatic Eddy covariance (AEC) technique. Sandra will work under the supervision of Profs. Karl Attard and Ronnie Glud.
AEC is a direct and non-invasive method that allows the quantification of benthic metabolism rates by measuring oxygen fluxes with a high resolution, during days or weeks and continuously.

The stay at SDU will allow us to transfer the know how to our laboratory, which only a few laboratories at international level have and extend our collaboration with our Danish colleagues. Furthermore, in practical terms, the use of the AEC method will facilitate the monitoring of benthic metabolism rates in different habitats in our area and to study the effect of extreme wind events, without the logistical problems associated with sampling under such conditions. Our new project MULTIFLUX will focus heavily on this aspect.